The page is constantly under reconstruction...
Research gate project:
ESIPT, and Fluoroborates - theory and experiment, see NSF project H09/39; 2016
Linked to the currently running
Former NSF project: H19/53 - 2017, Novel Intramolecular Proton Transfer
Heterocyclic Luminophores and Their Complexes for Modern Biomedicine
Contract # ДН 19/11, Dec. 10, 2017; Coordinator: Assoc. Prof. Dr. Snezhana Bakalova
Present NSF Contract # КП-06-Н59/1, 15.11.2021; Coordinator: Assoc. Prof. Dr. Snezhana Bakalova
First project Participants:
|
Prof. Vanya Kurteva
Assoc. Prof. Irena Philipova
Assoc. Prof. Vanya Mantareva
Assoc. Prof. Ivan Angelov
Prof. Jose Kaneti
Dr. Nadezhda Tabakova
Meliha Aliosman
Nina Stoyanova
Vera Assenova
Prof. Han Zuilhof (WUR, NL) |
Prof. George Miloshev
Prof. Milena Georgieva
Dr. Dessislava Staneva
Borislava Boteva
Bela Vasileva , |
Some ESIPT capable heterocyclic luminophores, used in the project
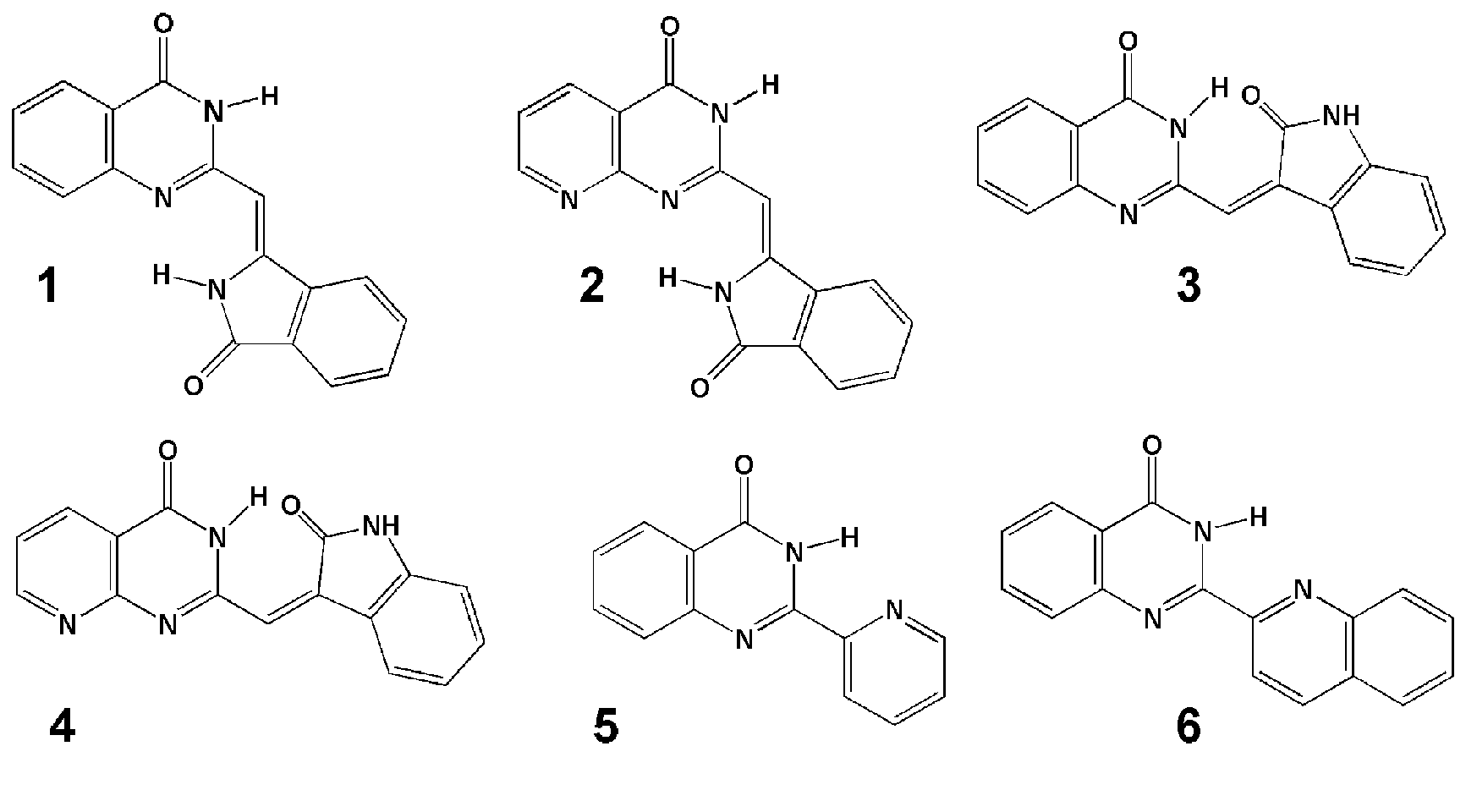
Obvious ESIPT candidates among the above molecules are only 1 and 2. 3 and 4 are internally hydrogen bound
even in their ground electronic states, while 5 and 6 are problematic. Nevertheless, the shown molecules are biologically
active due to a mechanism not involving hydrogen bonding. Literature data shows that this activity is possibly related to
association of quinazoline derivatives to specific nucleic acid secondary structures, guanine quadruplexes.
Our article on the above hypothesis has been published in BBA-General subjects.
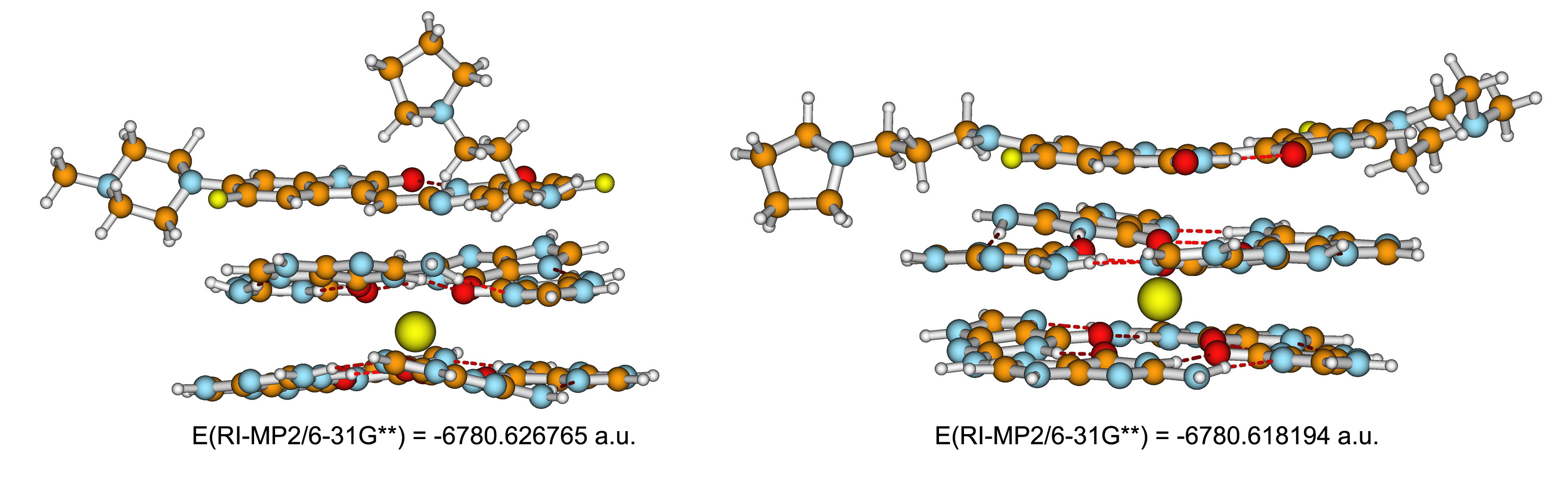
Two model drawings of a G4 complex with schizocommunin, molecule 3 above:
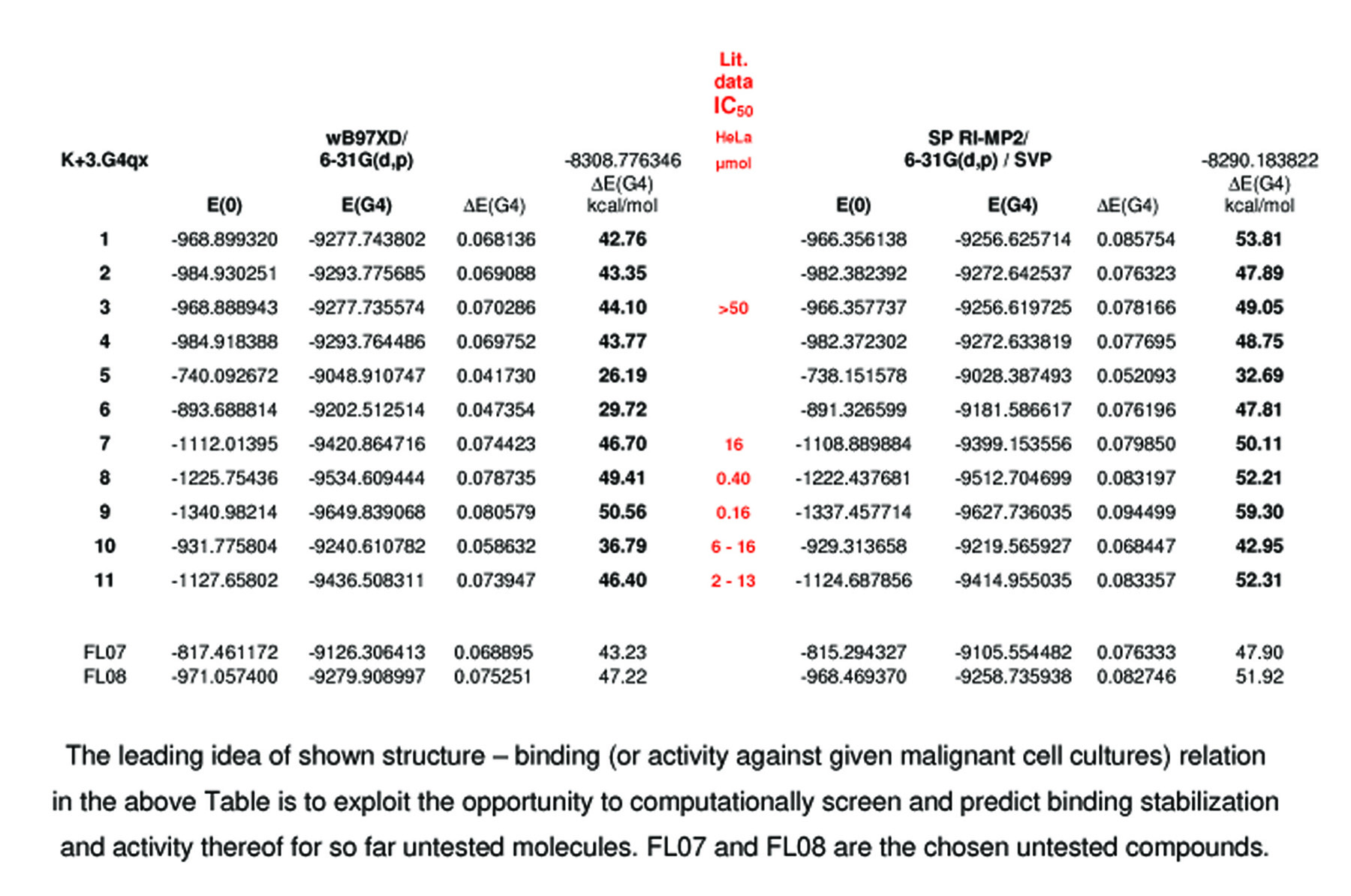
Our own computational and literature biological data are summarized in the following Table.
Compound numbering is taken from the above Scheme, and the published article.
A presentation on the 13th European Conference on Computational and Theoretical Chemistry
(EuCo-CTC 2021):recorded on the YouTube.
A couple of other presentations
e.g. on the Canarias by Milena introduce ligand affinity A(L.G4),
E(L) + E(G4) = E(L.G4) + A(L.G4),
from the computed energies E of ligands L, G4 quadruplexes, and their complexes L.G4 to show the following relationship:
QUARFLOXIN CX-3543: A(L) = -57.3 kcal/mol.............................UNDER Clinical trials
Compound P0108610048 from Otavachemicals: A(L) = -56.7 kcal/mol.......Prospective
Compound P0107740028 from Otavachemicals: A(L) = -42/7 kcal/mol.......Trash...
Also see Lecture Notes in Computer Science, 2022, 13346, pp. 1–11
We are about to yield to the QSAR temptation... and below is the graph we got.
QUARFLOXIN, with IC(50) in the nanomoles, should be to the far bottom left of the graph...
Moreover, we also won
another NSF grant to this end!:-)
Project ID: КП-06-Н59/1, 15.11.2021
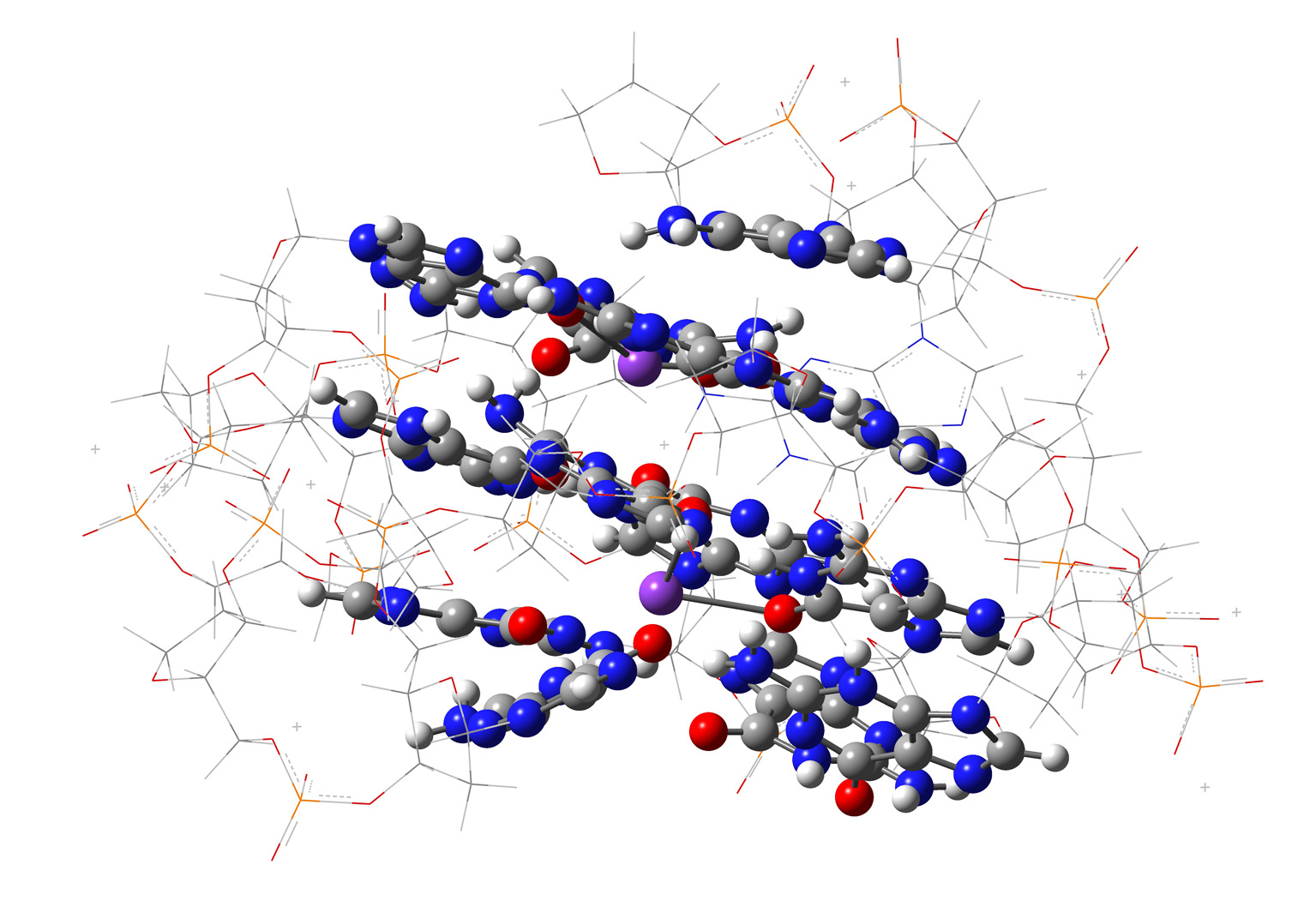
... on G4-ligand conformations ...
and RECOGNITION...
This is a link, where a further direction of G-quadruplex thought might be discussed:
in a special issue of IJMS, IF = 5.924 (2020 Journal Citation Reports)
Return to starting page or to the
IOCCP home page.






